Olivier Bardagot, Brandon T. DiTullio, Austin L. Jones, Justin Speregen, John R. Reynolds, Natalie Banerji
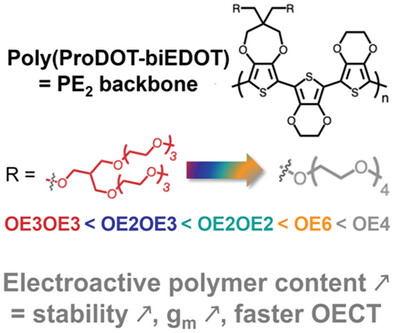
The development of devices based on organic electrochemical transistors (OECTs) relies on the rational design of high-performing organic mixed ionic-electronic conductors (OMIECs). Here, a series of solution-processable copolymers composed of unsubstituted 2,2′-bis-(3,4-ethylenedioxy)thiophene (biEDOT) and 3,4-propylenedioxythiophene (ProDOT) substituted with linear or branched oligo(ethylene oxy) (OE) side chains are reported. By varying the size of the linear and branched side chains, it is found that the highest OECT performance is achieved with a near equivalent molar mass of the side chain and electroactive conjugated polymer repeat unit. With four OE units (PE2-OE4,electroactive content of 49%), OECTs with state-of-the-art normalized transconductance (453 ± 70 S cm−1) and µC* (830 ± 37 F cm−1 V−1 s−1), rapid dedoping kinetics, and a pulsing stability of 99% IDS retention over 200 ON/OFF cycles are achieved. A consistent improvement in OECT stability with decreasing side-chain size is also observed. The origin of the enhanced stability is rationalized by correlating IDS losses to changes in channel absorbance cycle after cycle during operation. This work encourages the calculation of the electroactive polymer content of an OMIEC when designing the side chains. It also shows that the PE2 backbone with short OE side chains is a promising structure for (bio)electrochemical devices.